

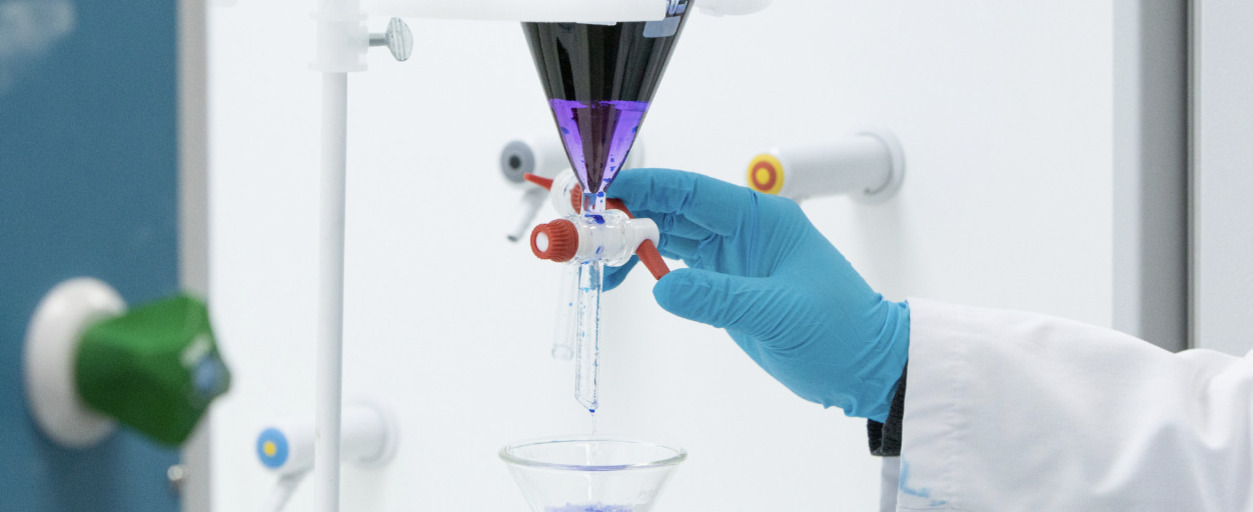
Noumed’s Quality Control facility is fully equipped for testing inwards materials and finished products.
The facility holds
High-spec Instrumentation
Elaborate Wet Lab for chemical analysis
Dedicated Stability Area with temperature and humidity chambers ranging from Zone I to Zone IV b
And is capable of
Supporting Manufacturing and Product Development
Carrying out Analytical Method Transfer in-house
Supporting routine testing of raw materials, finished products and stability samples


Quality Control analysts ensure that experiments are completed according to established standard operating procedures (SOPs), and also good laboratory practices (cGLP).

An MHRA accredited manufacturing facility manufactures multiple dosage forms and flexible batch sizes
Quality Control is fitted with high-spec analytical and testing equipment to support manufacturing and development
The fully equipped warehouse has provisions to store materials requiring cold storage conditions. It also has a dedicated area to store controlled substances
The focus on adhering to GMP guidelines and ensuring compliance to regulations, enables us to meet quality, efficacy and safety requirements. Through pharmacovigilance, product performance is regularly monitored to ensure patient safety
Noumed has a network of CMOs in various geographies to manufacture diverse dosage forms in addition to what can be manufactured in-house
